Abstract
Background: Metabolic reprogramming is a hallmark of malignancy, which is involved in tumorigenesis, deteriorate and clonal evolution. Current treatment regimens for ALL are L-asparaginase-intensified and multiagent antimetabolite-based therapy. However, there is still a lack of in-depth research on the metabolic characteristics of B-ALL and its relationship with drug sensitivity, molecular genetics, clinical prognosis and other fields.
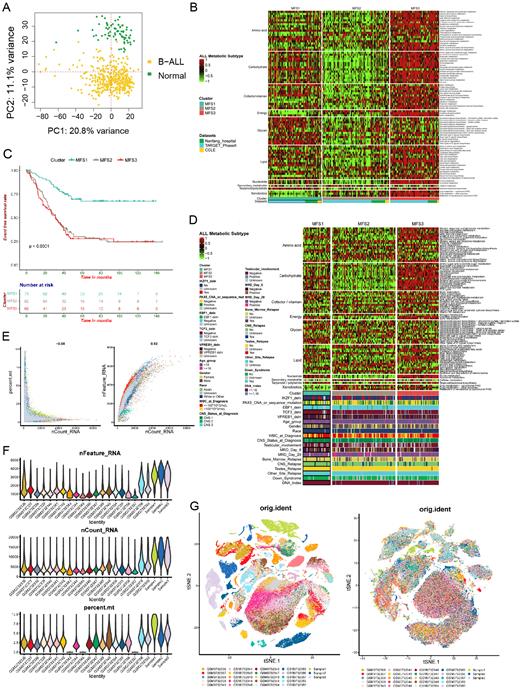
Methods: We utilized multi-omics data from Nanfang Hospital (the PDT-ALL-2016 cohort, n=112), as well as data from TARGET (n=187), GEO (n=921), CCLE (n=15), GTEx (n=70) and other databases, combined with in vitro cell function experiments, to establish B-ALL metabolic subtyping based on heterogeneous metabolic characteristics and explore metabolic targeting of metabolic fragility. First, the GSVA algorithm was used to evaluate the enrichment abundance of 85 metabolic pathways of 10 categories in 1215 B-ALL samples. Subsequently, unsupervised clustering was performed based on the enrichment abundance to separate B-ALL into heterogeneous subtypes with different metabolic features(MFSs); and meanwhile, WesternBlot, Seahorse, LC/MS and single-cell RNA sequencing were used to verify the metabolic characteristics of the MFSs from the fields of metabolic key enzymes, oxygen consumption rate (OCR), extracellular acidification rate (ECAR), metabolite abundance and metabolic pathway activities in single-cell level. Moreover, according to the metabolic preferences and dependencies of the MFSs, drug experiments of inhibitors targeting specific metabolic pathways were conducted in different MFSs cell lines, and combined with traditional chemotherapeutics to explore synergistic effects in specific MFS B-ALL.
Results: Our study showed that B-ALL could be separated into three metabolic-feature subtypes (MFSs): MFS1, the polysaccharide-metabolic subtype with upregulated glycan metabolism; MFS2, the cold metabolic subtype with downregulated of most metabolism. MFS3, the glycolytic subtype with upregulated of nucleotide and carbohydrate metabolism. The 5y-EFS rate of MFS1 was about 70%, and that of MFS2 and MFS3 were about 27%, which were significantly worse than that of MFS1. MFS2 and MFS3 had a higher proportion of patients with recurrence, CNS involvement and MRD positive. There were also differences in MICM subtypes among the three MFSs. In addition, different MFSs did indeed exist metabolic characteristics that matched to their metabolic preferences in terms of metabolic enzymes, OCR, ECAR and abundance of metabolites. Notably, MFS1 was most sensitive to the traditional chemotherapeutics such as asparaginase, dexamethasone, 6-MP and so on, while MFS2 was resistant to these chemotherapeutics; MFS3 showed sensitivity to inhibitors targeting glycolysis, and PKM2i (an inhibitors targeting glycolysis pathway) could reverse the resistance of MFS3 to asparaginase.
Conclusion: In summary, by integrating multi-center and multi-omics B-ALL data, our research innovatively established and verified a novel metabolic classification system of B-ALL, which revealed that metabolic preferences and dependencies have important prognostic significance in B-ALL. By deciphering metabolic characteristics and metabolic fragility of B-ALL, it is expected to achieve targeted therapy for the metabolic fragility and improve clinical prognosis.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal